Zeolite - Identifying Quality & Facts
First, a few answers to some basic questions about zeolite in general, which I hope will be as simple as possible.
What is zeolite clinoptilolite?
An aluminosilicate, a stone with a framework structure consisting of natural silicon and aluminium oxide with an extremely large number of cavities (micropores). These are tiny ‘passages’ with a diameter of 4-6 angstroms (0.4 - 0.6 nm). Because it is specially ground, one gram of it consists of thousands of small particles and has a very large specific surface area (inside and outside, including the micropores). This allows a large number of pollutants, such as heavy metals, ammonia, cadmium and the like, to attach themselves to these pores. It itself contains silicon and, among other things, calcium, potassium, sodium, magnesium and other mineral ions, all of which are negatively charged. There are many different types of zeolite, but only clinoptilolite zeolite has special properties and is used as an animal feed additive, for example, only if it has a clinoptilolite content of 80% or more. The name zeolite comes from the Greek ‘zeo’ for ‘to boil’ and “lith” for ‘stone’. The name goes back to the Swedish mineralogist Baron Axel Fredrick von Cronstedt. He held a blowtorch to the stone and it began to boil due to the release of water contained in the stone. Bentonite montmorillonite, also an aluminosilicate, was formed more slowly through the weathering of volcanic ash. Unlike zeolite, it is a layer silicate/clay silicate and therefore somewhat more difficult to mix with water. When simply stirred in, it sticks to the spoon like a lump of mud; it needs time to swell or must be mixed extremely finely with zeolite to separate the individual bentonite particles from each other so that they do not stick together. But anyone who has ever tried their hand at pottery knows this about clay. :-)
How was zeolite formed?
Millions of years ago, volcanic eruptions blew alkaline and alkaline earth metals and aluminosilicates out of the volcanoes in the form of ash, which fell onto salt water in the Carpathian Mountains, where the sea level was approximately 170 metres higher at that time. This caused a chemical reaction between the volcanic ash and the sodium-rich seawater, which ultimately led to the formation of natural zeolite.
How does zeolite work? How does zeolite work?
As a stone, it only passes through the (mammalian) body as a ‘guest’ and is not metabolised as such. Due to its negative charge, it attracts positively charged heavy metals and pollutants like a magnet and binds them to itself as it passes through the digestive tract until it leaves the body. In return for the pollutants it attracts, it releases its mineral ions and, not to forget, its silicon ions in colloidal form to the body. This is why it is referred to as an ion exchanger. In addition, the diameter of its micropores, at 4-6 angstroms, is very well suited for ‘docking’ heavy metal ions.
What does zeolite contain, what is zeolite made of?
Zeolite consists of silicon and aluminium oxide bound in a framework, and the aluminium cannot simply be dissolved out, as is often claimed. In technical terms, it works at temperatures above 400°C, but this completely destroys the framework structure. There are also repeated claims that grinding to less than 5.10 or 20 µm would ‘grind out’ the aluminium from the framework structure, but this is not the case. It is a molecular framework structure that would have to be completely destroyed, and that would only be possible below the nanoscale. The micropores (passages) in zeolite have an average size of 0.4 to 0.6 nanometres and are enclosed by the framework structure. That is a size ratio similar to that between a football and a speck of dust. But the essential thing is zeolite. Clinoptilolite consists of approximately 70% silicon, and its micropores contain crystal water with many mineral ions, which it releases to the body in exchange for harmful substances. These are metabolised directly and without conversion and help the body to help itself.
Silicon
But the most important thing is the mineral ions and, in particular, the natural colloidal silicon in zeolite. Silicon is actually the mastermind of our metabolism. Among other things, it regulates the electrolyte balance, which is the basis of all bioelectrical processes in the body, and much, much more. According to Prof. Hecht, colloidal silicon is the most bioavailable form of silicon for living organisms. The body can absorb it directly in this form without any conversion. Unfortunately, the silicon contained in plants is not so easy for the body to metabolise due to the size of the molecules.
It is not without reason that Prof. Dr. Karl Hecht named his first book ‘Clinoptilolite Zeolite - Silicon Minerals and Health’. Here is just one quote from the Kurzinformation_Wirkung_Klinoptilolith_Prof_Hecht.pdf
"What does SiO2 mean for humans? SiO2, the biogenically influenced primordial mineral of all living beings
The main function of natural clinoptilolite zeolite is performed by SiO2, also known as silicic acid, which is supplied to the human body in colloidal form.
Silicon is known to be the second most abundant element on our planet after oxygen. SiO2 accounts for the majority of this.
SiO2, which is found not only in natural clinoptilolite zeolite, but also in clay and montmorillonite as well as in many plants (e.g. horsetail, bamboo, nettle, conifers), is the oldest healing and cosmetic remedy known to mankind.
Although there is a wealth of scientific literature on the subject, few doctors or other healthcare professionals in Germany are aware of the effects of SiO2 today.
In 1975, the Russian research group led by M. G. Voronkov published a book entitled ‘Silicon and Life’ in German. It cites over 5,000 scientific sources. Between 1970 and 1986, the American silicon researcher E. M. Carlisle published many scientific findings. In 1986, the Ciba Foundation held a silicon symposium, and a conference proceedings volume was published.
© Prof. em. Prof. Dr. med. habil. Karl Hecht 29 Silicon dioxide is the basic element of life. Without SiO2, no life processes, no growth and no bioelectricity are possible. Organisms that are deficient in silicon age and become ill quickly.
What is special about silicon in zeolite or bentonite is the way in which it is made available to the body. For example, the human body can only absorb 30-40 mg of silicon per day, but only if it is provided in colloidal form.
The body has to convert silicon supplements so many times that in the end there is virtually nothing left to metabolise. The multiple conversions are a real challenge for the body until it finally has colloidal silicon dioxide. And only then can it absorb 30-40 mg per day.
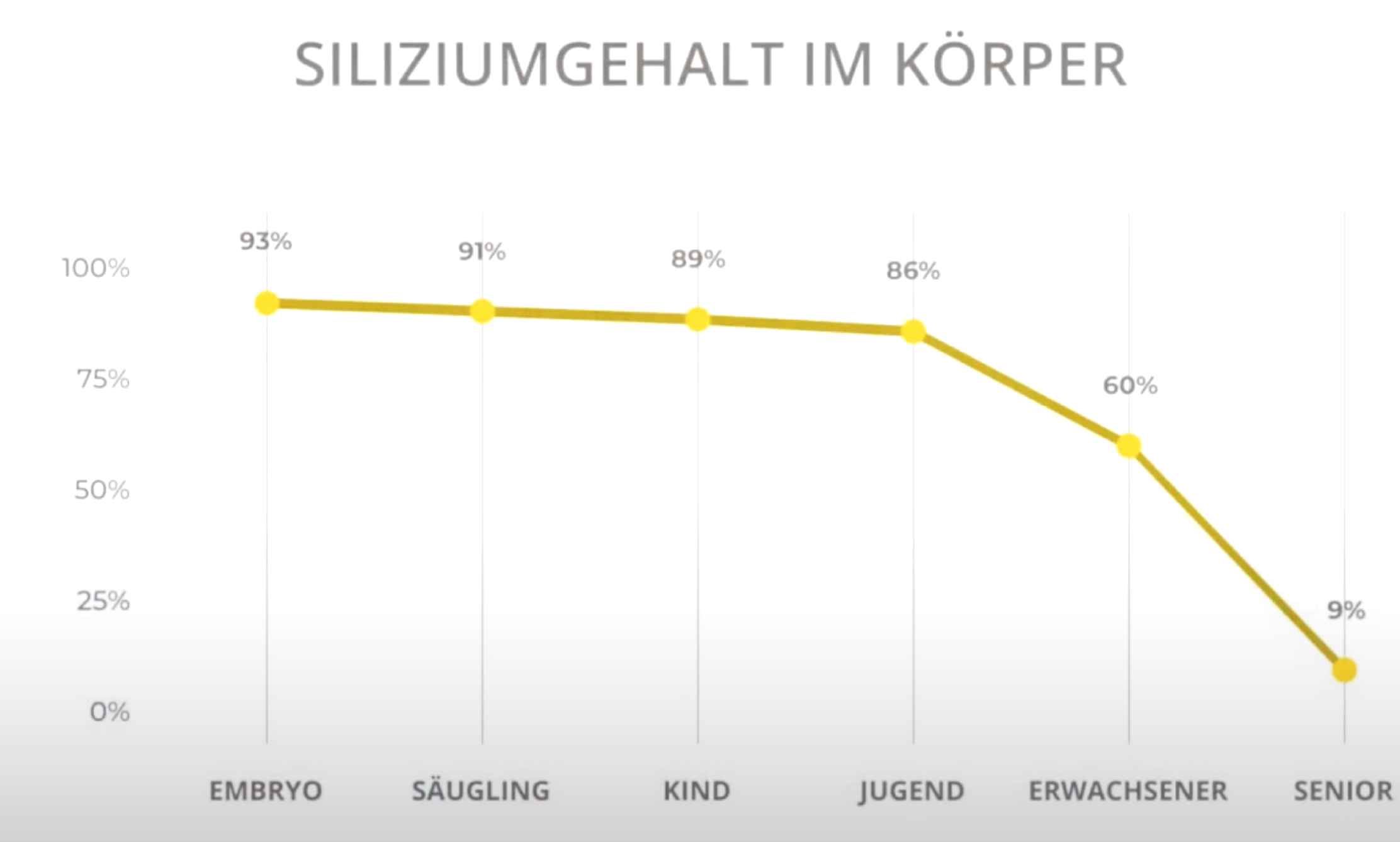
However, it can send colloidal silicon directly to where it is needed in the body, e.g. to repair inflammatory processes or wounds. It is not without reason that silicon is referred to as the ‘body's fire brigade’. The special thing about silicon is that the amount in the body continues to decrease in every living being from birth onwards. In fact, this process begins even before birth, as foetuses have higher silicon levels than infants. From then on, the levels decline throughout life.
Another very special feature of silicon is the biological transmutations described by Kervran. He proved that biological transmutations of minerals take place in the nuclei of atoms.
He was the one who discovered that chickens that were fed without calcium but had mica, feldspar and the like to peck at still laid eggs with a beautiful, firm shell, and he wondered how this was possible without calcium. He then discovered that calcium can be formed from silicon, potassium and magnesium as part of biological transmutation. This also explains Prof. Hecht's statement as to why silicon could contribute to significantly faster bone healing than calcium in the case of bone fractures, especially in the lower extremities.
Below are two illustrations showing silicon in the body according to age and a second showing silicon levels with and without zeolite. However, it should be noted that it takes years for silicon levels in the body to rise again, but then they continue to rise steadily.

Why not use a metal spoon?
Zeolite does not work chemically, but physically/electrically in the body. Since water and metal spoons are both electrically conductive media, and zeolite contains a large number of negatively charged (mineral) ions, a second electrically conductive medium in the water would weaken it. Stirring vigorously and persistently in water increases the electricity of the zeolite in the water when a plastic, porcelain, wooden or glass spoon is used. Prof. Hecht has demonstrated this very clearly on several occasions. When dry, it is no problem to use a metal spoon to transfer the zeolite to another container, for example, as there is no water involved. It is also very useful to stir the zeolite in the storage container a little before removing it, as the friction between the particles increases the electricity enormously. It is not necessary to stir the entire container, just the area where the zeolite is to be removed. Due to its fine grinding, it settles and becomes looser and lighter when stirred.
What about the shelf life of zeolite?
As far as the shelf life of zeolite is concerned, it is actually infinite if the container is tightly sealed. Geologically speaking, zeolite is already millions of years old and, as a stone, cannot ‘rot’. In terms of its power, it can only slowly wear away in the air, becoming weaker bit by bit. However, this does not happen very quickly, otherwise there would be no effective zeolite left in the open-cast quarries. If, for example, you spread a tablespoon of zeolite on a saucer, it will still take months for it to be depleted through cation exchange with the air. Zeolite has the ability to ‘clean’ its immediate environment through cation exchange, which it does in water, air, digestive tracts, simply anywhere you put it. It works at full capacity at a pH value of approx. 8.2 to 8.3 and a temperature of approx. 38° Celsius, i.e. roughly speaking in a warm, alkaline, wet environment. It works much more slowly in the air and, because it is dry, does not have any direct cation exchange possibilities, so everything happens in slow motion, so to speak. Zeolite is also used in agriculture as a feed additive to neutralise mycotoxins in animal feed such as silage and the like as soon as they are fed, which means that zeolite renders harmful germs harmless rather than allowing them to infect the animal. In short, zeolite that is several years old may become slightly weaker if it has not been sealed carefully, but that is all.
What is the deal with the lead in zeolite?
Zeolite is a stone that is not metabolised and, like all aluminosilicates, it contains heavy metals. A stone that has an affinity for lead logically also contains lead to a certain extent. It has absorbed it from its environment over thousands of years. The average lead content of the earth's crust is 15 ppm (mg) lead per kg, excluding arable land, of course, which contains far more. A zeolite that is able to bind lead to itself through Van der Waals forces cannot remain completely free of lead over thousands of years, unless it is completely isolated or has no affinity for lead at all. However, in that case, it cannot be used effectively for lead removal. Cuban zeolite, for example, usually contains very little lead, but it also has no great affinity for lead, only for copper, which is why it is very often used for wastewater and agricultural soil remediation. However, it is not known to absorb particularly large amounts of lead.
With regard to zeolite clinoptilolite, I would like to address a general prejudice that is repeatedly published. This is not and should not be considered a recommendation for consumption. Zeolite clinoptilolite is not a dietary supplement. It passes through the body as a guest, binding harmful ions to itself and in return releasing mineral ions in colloidal form to the body. These are metabolised, but not the zeolite.
If a food (supplement) containing 3 ppm lead/kg (maximum value for food supplements) is consumed or fed, it is completely bioavailable and the body absorbs 3 ppm lead, which it must process somehow.
When zeolite clinoptilolite is consumed or fed, the heavy metals present in the zeolite are bound by Van der Waals forces and can only be dissolved by certain acids. This has been scientifically researched and confirmed in laboratory tests.
When zeolite is tested for lead, this is done using aqua regia or microwave digestion. Aqua regia is a mixture of concentrated hydrochloric acid and concentrated nitric acid in a ratio of 3 to 1 and is considered to be pretty much the strongest acid there is.
Laboratory testing of a zeolite revealed a lead content of 9.7 mg lead/kg. In my opinion, this is a good value in the lower range.
However, if instead of aqua regia, an acid with a pH value of 1.5 is used, which corresponds to the stomach acid of non-vegetarian mammals, the bioavailability values are completely different. With 5 g of zeolite in 1 litre of this artificial stomach acid at 40°C and a retention time of 30 minutes, the result is a value of 0.008 ppm, which, converted to 1 kilogram of zeolite, results in a value of 1.6 mg of lead per kg as bioavailable in the stomach. And this is not in a food or dietary supplement, but in a stone and ion exchanger that does not readily release heavy metals voluntarily. In addition, in the duodenum at a pH value of approximately 8.2, it is able to absorb considerably more lead than the amount it has released to the gastric acid. Its cation exchange capacity is determined including the lead it contains. Only in the alkaline environment of the intestine does zeolite-clinoptilolite unfold its power of adsorption. This is precisely what makes zeolite-clinoptilolite so special, in stark contrast to activated carbon, which, once saturated, releases everything again.
However, I have also seen suppliers who seem to want to suggest that there are no heavy metals in aluminosilicates at all. Statements such as ‘Tested in independent laboratories, therefore free of harmful substances’ are utter nonsense. Testing definitely does not change the heavy metal content, but what wouldn't you do for marketing? :-))
For your information:
Maximum values for total heavy metals in medical zeolite class I, IIa / IIb: 50 ppm / kg
Maximum value for lead in zeolite as an animal feed additive: 60 ppm / kg
What does this have to do with certification as a medical device?
It would actually be quite simple if it weren't for a complex political and legal situation in the EU. It goes back to a court case at the turn of the millennium, when a company wanted to market zeolite as a dietary supplement and was sued. (To explain, zeolite cannot simply be considered a dietary supplement because it is not metabolised, but only passes through the body as a guest. Of course, it releases its mineral ions to the body in exchange for pollutant ions, but zeolite as a stone cannot be metabolised.
This court case cost the defendant millions, in German marks at the time, so they gave up. Shortly after the end of the trial, however, there was a successful effort in the EU to put this stone, which cannot be a dietary supplement or a foodstuff, on a list of novel and newly invented foods, the so-called Novel Food List. This list (link https://food.ec.europa.eu/food-safety/novel-food/authorisations/union-list-novel-foods_en) contains ‘novel foods’ that retailers are not allowed to publicly recommend for human consumption.
"What are novel foods?
The term ‘novel food’ refers to any food that was not used for human consumption to a significant degree in the European Union before 15 May 1997 and that falls into at least one of the categories listed in Article 3 of the Novel Food Regulation () 2015/2283."
This led to the idea of certification as a Class I, IIa or IIb medical device (formerly medical aid). The Hartmann company had already paved the way for this by certifying zeolite for medical dressings. Class I is purely external, but goes into the mouth without being swallowed. Class IIa can also be taken internally, but for a maximum of 30 days. A waiting period of 5 days was calculated until the zeolite has finally left the body, which means that some suppliers recommend treatments lasting exactly 25 days. :-)) Class IIb means unlimited use, which of course most have also certified. This makes it possible to recommend its use by humans in a striking manner. With such certification (link to the three classes with explanations, https://flexikon.doccheck.com/de/Medizinprodukt), the maximum value of 50 ppm/kg total heavy metal content is tested, and then there is microbiology, which is max. 2000 KbE/g, which can certainly be exceeded in the case of a stone from a quarry. The only strange thing about this regulation is that zeolite, when working in its alkaline environment, neutralises and binds to germs that generally prefer and produce an acidic environment. When I consider that breakfast cereal is allowed to contain a maximum total germ count of 100,000 KBE/g...
Certified items from our everyday lives include FFP2 masks, dental implants, stents, breast implants, etc. (list is very incomplete).
Certification as a medical device is not necessarily a guarantee of good product quality and/or safety. Even non-certified zeolite products can meet the highest quality criteria; anyone can have their batches tested in laboratories, even if they are not obliged to do so. Non-certified products are usually less expensive, as there is no need for extensive documentation with QM, product master files, descriptions of internal quality assurance processes, external auditors and a string of additional projects and costs.
This is confirmed by the many scandals involving certified medical devices such as implants, prostheses, pacemakers and countless ‘best-tested drugs’ that were withdrawn from the market after a very short time without fanfare due to massive side effects.
What certification does not seem to place any greater value on is cation exchange capacity, grinding, with or without grinding media, particle size distribution and selectivity series. Detailed data sheets with all the information are also no longer necessary; the CE mark replaces all of this.
In any case, no one can take away an adult's personal responsibility.
The data sheet
The data sheet should contain all relevant information, not just the mineralogical composition. Prof. Hecht has always insisted that data such as selectivity series, grain size, clinoptilolite content, silicon-aluminium ratio, cation exchange capacity (CEC), heavy metals and, if possible, one-off further investigations of the quarry should be listed. These are not all continuous investigations, but the heavy metals should be examined for each batch.
Zeolite quality
Let's take a more relaxed approach after these somewhat hysterical discussions on the internet about what supposedly constitutes a good zeolite. From the well-known (without data sheet) and unprovable ‘best quality, finely ground to ’the grind size must not be less than 100 microns, otherwise nano-particles will form!!" we should take a closer look at what really defines quality.
You don't have to be an expert to understand what matters.
A small, simple example: when you buy high-quality wall paint, you make sure that one bucket of paint will cover 100 m² and not just 15-20 m². That would not only be expensive, but also exhausting.
Fortunately, there is a key figure that specifically indicates how much ‘power’ a zeolite has, and that is the cation exchange capacity (CEC). It describes how many cations the zeolite can absorb. This means that since cations are positively charged, such as various toxins and heavy metals, the higher the CAC, the higher the absorption capacity. A high CAC is always accompanied by fine grinding and effective activation, whether by grinding in an air stream or thermal activation. The specific surface area is also closely related to this. Since finer zeolite is very light, one kilogram of 6 µm material, for example, has a volume of 2 litres. You certainly pay more per kilogram than for a coarser material, but you get more of it in terms of (volume) quantity.
An example as a reference point: Identical zeolite, different grinding and grain sizes
A 1000 ml can with zeolite d50% 6 µm = 500g - bulk density 500 g / litre - CAK approx. 210 meq / 100 g
A 1000 ml can with zeolite d50% 28 µm = 750 g - bulk density 750 g/litre - CAC approx. 125 meq/100 g
Here in a little more detail:
GRINDING IS THE DECIDING FACTOR
It all starts in the quarry, of course, where the zeolite is ‘broken down’ to a grain size of 10-50 mm and then pre-ground for us in a ring roller mill (but there are many others) to a grain size of between 200 and 300 µm. It could also be ground to a smaller size, saving the cost of additional jet mill grinding, which is what most suppliers do. Any supplier who bears the cost of final grinding in an air stream will also mention this in their product descriptions.
There is also the ball mill, but this would be more suitable for bentonite montmorillonite, which is around 10 µm and is hot-ground in a ball mill and dried at the same time.
This is where the journey ends for most zeolites, and they are sent for distribution.
But for our zeolite (and, of course, all other truly tribomechanically activated zeolites (TMAZ)), this is where the real work begins. It comes out of the ring roller mill at approx. 200-300 µm and is sent to our specialised service provider, where it is further processed in a plant whose ‘heart’ is a spiral jet mill with a classifier, thereby truly activating it. This takes place in a precisely controlled air flow at approx. 300 km/h.
Here, the air is fed into the grinding chamber in a ring shape via several tangentially arranged nozzles (the air flow can be imagined as helical), whereby the air reaches a very high speed. The zeolite is captured by the air jets, accelerated and crushed by particle-particle friction until it has exactly the desired size, which is controlled by a so-called classifier. The particles of the correct size are ‘ejected’, as are particles that are too large. Finally, our zeolite comes out of the mill in exactly the specified grain sizes, but now activated, as can be clearly seen from the values in the data sheet and in the diagram:
Specific surface area 400-600 m²/g, cation exchange value 210 meq/100g. It now has an average grain size of 6µm, less than 3% have a maximum grain size of over 20 µm, no particles over 30 µm, and nanoparticles can be largely ruled out with this process.
These are some of the key data you need to look for from all suppliers in order to recognise what is being offered to you:
- Grinding, was it ground in an air stream?
- Cation exchange capacity: 150-200 meq/100g is excellent, e.g. 200 meq/100g – 200 mval/100g – 200 cmol/kg are the units that roughly correspond
- Particle size: Top cut, average particle size should be precisely quantified or include a diagram
- Specific surface area: depends on the grinding process; the finer the grind, the larger the surface area. The surface area of the micropores is important as it indicates the porosity of the material
- Clinoptilolite content: the higher the better, up to 95% possible, but not essential
- Silicon content: anything around 70% is very good
- Bulk density: properly ground zeolite is very light, 500 g could correspond to 1000 ml
- Silicon/aluminium ratio: 5:1 is good
Anyone who offers zeolite actually has a data sheet for their mineral with information that provides insight into the average quality of the mineral. Anyone who does not publish this at all or only in part either does not know any better or has their reasons. Zeolite that has been ground to 100 to 150 (microns), for example, cannot deliver the same cation exchange values as zeolite that has been ground to 6µm (microns) in an air stream. This is mainly because the surface area per square metre is not comparable for coarse material and, of course, there is no significant static charge.
The claims made by some suppliers that they do not grind so finely because of the formation of nanoparticles must be countered by the fact that these do not occur in quantifiable quantities during high-quality grinding. 1 µm (micron) corresponds to 1000 nm (nanometres). That is quite a big difference; after all, a metre is not simply equated with a kilometre.
Grinding (activation, micronisation) remains the key to cation exchange capacity and specific surface area. Our zeolite is activated and micronised as a service by the manufacturer of Jetmills and now also by a second specialised service provider with an identical mill, which is why we know we are in very good hands ‘at the source of the technology’. In addition to the quality of the mineral itself, only the technology and knowledge of micronisation and activation play a decisive role.
One important factor in the raw material is the clinoptilolite content. This is where the first internet legends begin: ‘There is no zeolite in the world with a 95% clinoptilolite content!’ (quote) This is not true, but the total silicon content is considerably more important. This applies equally to zeolites such as bentonites and determines how much silicon can be released in ion exchange.
Then there is the cation exchange capacity, or CEC for short, which is higher the better, ranging from 50-60 meq/100g for some zeolites to no less than 150-160 meq/100g for really high-quality ones. The finer the grinding, the higher the CEC.
The specific surface area is very important, normally measured in square metres per gram. There are some ranging from 20 m²/g to 60-70 m²/g. However, to speak of very high-quality material, one should look for values not below 250 m²/g if one wants a high-quality ground zeolite. However, these tests are complex and costly.
The silicon/aluminium ratio, naturally as much silicon and as little aluminium as possible, from 4:1 (absolute minimum according to Prof. Dr. Hecht) up to anything higher than 5:1 is in the very good range.
Normally, the silicon content of a good zeolite is 60%-70%, while the aluminium content can be between 10%-16%. (Source: Prof. Dr. Hecht et al.)
The average particle diameter is often simply given in sizes such as 0 - 150µm (microns), which unfortunately is not helpful and rather indicates that the exact particle size distribution may not be known because it has not been ground in a controlled manner or tested. The average particle size d 50 normally means a minimum of 50% of the material. The top cut is the largest particle size contained, e.g. 2% with 24 µm (microns). We then know for certain that at least 50% of the material is 6 µm (microns), but a maximum of 2% is 24 µm (microns). The material does not contain anything larger than this maximum 2% with 24 microns. What we generally provide as information is a chart of the entire particle size distribution. This is the best way to see what quality you are dealing with.
There is another point worth knowing: zeolite does indeed work immediately when it comes into contact with air, but not to such an extent that you need to worry about it losing its potency instantly. It only really gets going when it encounters an environment that offers it real ion exchange tasks, i.e. in liquids or similar. The most important thing is to seal the containers well so that no air or moisture can penetrate, then it will retain its properties for years. Zeolite has no problems with sunlight at all.
A few words about test reports. We have already been test winners, comparison winners and price-performance winners, but it must be borne in mind that these portals cannot carry out real laboratory tests. This would not be financially viable because the individual affiliate commission earnings simply do not generate enough profit to even begin to finance a laboratory test. We worked with three different laboratories for approximately six months to conduct the initial basic tests on our zeolite in order to know exactly what we wanted to offer. For a comparison portal, even our testing of each batch for heavy metals is too costly. So they simply compile whatever they can find, and in some texts you can tell that they don't have a great deal of knowledge about the subject matter behind it.
We hope that our little digression will be of some help. Of course, we do not claim to be complete or error-free, and we welcome any suggestions you may have.
